1 Heikkinen T, Järvinen A. The common cold. Lancet. 2003 Jan 4;361(9351):51–9.
2 Germann M, Hilfiker A, Huber BM, Rosamilia C, Grandinetti T, Wingeier B, et al. Immunstimulation zur Prävention und Therapie von akuten Luftwegsinfektionen. Primary and Hospital Care. 2019 Nov 6;19(11):345–9.
3 Hooton TM. Uncomplicated Urinary Tract Infection. N Engl J Med. 2012 Mar 15;366(11):1028–37.
4 Kranz J, Schmidt S, Lebert C, Schneidewind L, Mandraka F, Kunze M, et al. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients: Part 1. Urologia Internationalis. 2018;100(3):263–70.
5 Heit JA. The Epidemiology of Venous Thromboembolism in the Community. Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):370–2.
6 Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. The American Journal of Medicine. 2004 Jul;117(1):19–25.
7 Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Journal of Thrombosis and Haemostasis, 15: 242–245. 2006;367:5.
8 Schmidt M, Horvath-Puho E, Thomsen RW, Smeeth L, Sørensen HT. Acute infections and venous thromboembolism: Infections and VTE. Journal of Internal Medicine. 2012 Jun;271(6):608–18.
9 Grimnes G, Isaksen T, Tichelaar YIGV, Brækkan SK, Hansen J-B. Acute infection as a trigger for incident venous thromboembolism: Results from a population-based case-crossover study. Research and Practice in Thrombosis and Haemostasis. 2018;2(1):85–92.
10 Clayton TC, Gaskin M, Meade TW. Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practice database. International Journal of Epidemiology. 2011 Jun 1;40(3):819–27.
11 Cowan LT, Lutsey PL, Pankow JS, Cushman M, Folsom AR. Hospitalization with Infection and Incident Venous Thromboembolism: The ARIC Study. Thromb Res. 2017 Mar;151:74–8.
12 Ribeiro DD, Lijfering WM, Vlieg AVH, Rosendaal FR, Cannegieter SC. Pneumonia and risk of venous thrombosis: results from the MEGA study. Journal of Thrombosis and Haemostasis. 2012;10(6):1179–82.
13 Samama M-M. An Epidemiologic Study of Risk Factors for Deep Vein Thrombosis in Medical Outpatients: The Sirius Study. Arch Intern Med. 2000 Dec 11;160(22):3415–20.
14 Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing G-J, Kyrle PA, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480–3.
15 White RH. Identifying Risk Factors for Venous Thromboembolism. Circulation. 2012 May;125(17):2051–3.
16 Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016 Feb 1;149(2):315–52.
17 Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Advances. 2020 Oct 13;4(19):4693–738.
18 Bagot CN, Arya R. Virchow and his triad: a question of attribution. British Journal of Haematology. 2008 Oct;143(2):180–90.
19 Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. The Lancet. 1997 Dec;350(9094):1795–8.
20 Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells Score for Deep Vein Thrombosis in the Inpatient Setting. JAMA Intern Med. 2015 Jul 1;175(7):1112.
21 Engelberger RP, Aujesky D, Calanca L, Staeger P, Hugli O, Mazzolai L. Comparison of the diagnostic performance of the original and modified Wells score in inpatients and outpatients with suspected deep vein thrombosis. Thromb Res. 2011 Jun;127(6):535–9.
22 Wells PS. Does This Patient Have Deep Vein Thrombosis? JAMA. 2006 Jan 11;295(2):199.
23 Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk Assessment of Recurrence in Patients With Unprovoked Deep Vein Thrombosis or Pulmonary Embolism: The Vienna Prediction Model. Circulation. 2010 Apr 13;121(14):1630–6.
24 Hendriksen JMT, Geersing G-J, Lucassen WAM, Erkens PMG, Stoffers HEJH, van Weert HCPM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015 Sep 8;351:h4438.
25 Shen J-H, Chen H-L, Chen J-R, Xing J-L, Gu P, Zhu B-F. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016 Apr;41(3):482–92.
26 Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). European Heart Journal. 2020 Jan 21;41(4):543–603.
27 Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008 Jun;133(6 Suppl):381S-453S.
28 Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010 Nov;8(11):2450–7.
29 Anderson Frederick A., Spencer Frederick A. Risk Factors for Venous Thromboembolism. Circulation. 2003 Jun 17;107(23_suppl_1):I–9.
30 Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015 Jun;113(6):1176–83.
31 Eekhoff EMW, Rosendaal FR, Vandenbroucke JP. Minor Events and the Risk of Deep Venous Thrombosis. Thrombosis and Haemostasis. 2000;83(03):408–11.
32 Stralen KJ van, Rosendaal FR, Doggen CJM. Minor Injuries as a Risk Factor for Venous Thrombosis. Arch Intern Med. 2008 Jan 14;168(1):21–6.
33 Iorio A, Kearon C, Filippucci E, Marcucci M, Macura A, Pengo V, et al. Risk of Recurrence After a First Episode of Symptomatic Venous Thromboembolism Provoked by a Transient Risk Factor: A Systematic Review. Arch Intern Med. 2010 Oct 25;170(19):1710–6.
34 Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. The Lancet. 2003 Aug;362(9383):523–6.
35 Hendriksen JMT, Geersing G-J, Lucassen WAM, Erkens PMG, Stoffers HEJH, van Weert HCPM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015 Sep 8;351.
36 Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. The Lancet. 1997 Dec;350(9094):1795–8.
37 Sermsathanasawadi N, Suparatchatpun P, Pumpuang T, Hongku K, Chinsakchai K, Wongwanit C, et al. Comparison of clinical prediction scores for the diagnosis of deep vein thrombosis in unselected population of outpatients and inpatients. Phlebology. 2015 Aug;30(7):469–74.
38 Cornuz J, Ghali WA, Hayoz D, Stoianov R, Depairon M, Yersin B. Clinical prediction of deep venous thrombosis using two risk assessment methods in combination with rapid quantitative D-dimer testing. The American Journal of Medicine. 2002 Feb;112(3):198–203.
39 Penaloza A, Melot C, Motte S. Comparison of the Wells score with the simplified revised Geneva score for assessing pretest probability of pulmonary embolism. Thrombosis Research. 2011 Feb;127(2):81–4.
40 Neppelenbroek SIM, Rootjes PA, Boxhoorn L, Wagenaar JFP, Simsek S, Stam F. Cytomegalovirus-associated thrombosis. The Netherlands Journal of Medicine. 2018;76(5):4.
41 Justo D, Finn T, Atzmony L, Guy N, Steinvil A. Thrombosis associated with acute cytomegalovirus infection: A meta-analysis. European Journal of Internal Medicine. 2011 Apr 1;22(2):195–9.
42 Wegner I, Beer JH, Zobrist M. Eine ungewöhnliche Komplikation nach EBV-Infektion. Schweiz Med Forum 2012;12(17):355-356:2.
43 Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD. Pandemic H1N1 Influenza Infection and Vascular Thrombosis. Clin Infect Dis. 2011 Jan 15;52(2):e14–7.
44 Collins MH, McGinn MK, Weber DJ. Mesenteric Thrombosis Complicating Influenza B Infection. The American Journal of Medicine. 2016 Jun;129(6):e17–8.
45 Tichelaar YIGV, Kluin-Nelemans HJC, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb Haemost. 2012 May;107(5):827–37.
46 Wang C-C, Chang C-T, Lin C-L, Lin I-C, Kao C-H. Hepatitis C Virus Infection Associated With an Increased Risk of Deep Vein Thrombosis: A Population-Based Cohort Study. Medicine. 2015 Sep;94(38):e1585.
47 Ambrosino P, Tarantino L, Criscuolo L, Nasto A, Celentano A, Di Minno MND. The risk of venous thromboembolism in patients with hepatitis C. A systematic review and meta-analysis. Thromb Haemost. 2016 Oct 28;116(5):958–66.
48 Driesen Y, Verweij M, De Maeseneer M, De Dooy J, Wojciechowski M, Van Den Akker M. Vascular Complications of Varicella: Description of 4 Cases and a Review of Literature. The Pediatric Infectious Disease Journal. 2015 Nov;34(11):1256.
49 Howard JFB, Rokx C, Smit C, Wit FWNM, Pieterman ED, Meijer K, et al. Incidence of a first venous thrombotic event in people with HIV in the Netherlands: a retrospective cohort study. Lancet HIV. 2019 Mar;6(3):e173–81.
50 Mejer N, Westh H, Schønheyder HC, Jensen AG, Larsen AR, Skov R, et al. Increased risk of venous thromboembolism within the first year after Staphylococcus aureus bacteraemia: a nationwide observational matched cohort study. Journal of Internal Medicine. 2014;275(4):387–97.
51 Chen Y-G, Lin T-Y, Huang W-Y, Lin C-L, Dai M-S, Kao C-H. Association between pneumococcal pneumonia and venous thromboembolism in hospitalized patients: A nationwide population-based study. Respirology. 2015;20(5):799–804.
52 Rüegger K, Tarr P, Karatolios K, Humburg J, Hügli R, Jeanneret C. Brucellosis and thrombosis of the inferior vena cava. Vasa. 2017 Jan;46(1):60–3.
53 Dentan C, Epaulard O, Seynaeve D, Genty C, Bosson J-L. Active Tuberculosis and Venous Thromboembolism: Association According to International Classification of Diseases, Ninth Revision Hospital Discharge Diagnosis Codes. Clinical Infectious Diseases. 2014 Feb 15;58(4):495–501.
54 Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, et al. Risk Factors for Venous Thromboembolism in Hospitalized Patients With Acute Medical Illness. ARCH INTERN MED. 2004;164:6.
55 Tichelaar YIGV, Knol HM, Mulder AB, Kluin-Nelemans JC, Lijfering WM. Association between deep vein thrombosis and transient inflammatory signs and symptoms: a case-control study: Letters to the Editor. Journal of Thrombosis and Haemostasis. 2010 Aug;8(8):1874–6.
56 Monn MF, Hui X, Lau BD, Streiff M, Haut ER, Wick EC, et al. Infection and Venous Thromboembolism in Patients Undergoing Colorectal Surgery: What Is the Relationship? Dis Colon Rectum. 2014 Apr;57(4):497–505.
57 Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. Journal of Thrombosis and Haemostasis. 2014;12(9):1391–400.
58 Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. Journal of Vascular Surgery. 2007 Feb;45(2):335-342.e1.
59 Cohoon KP, Ashrani AA, Crusan DJ, Petterson TM, Bailey KR, Heit JA. Is Infection an Independent Risk Factor for Venous Thromboembolism? A Population-based Case-control Study. Am J Med. 2018 Mar;131(3):307-316.e2.
60 Rogers MAM, Levine DA, Blumberg N, Flanders SA, Chopra V, Langa KM. Triggers of Hospitalization for Venous Thromboembolism. Circulation. 2012 May;125(17):2092–9.
61 Abgueguen P, Delbos V, Chennebault JM, Payan C, Pichard E. Vascular Thrombosis and Acute Cytomegalovirus Infection in Immunocompetent Patients: Report of 2 Cases and Literature Review. Clinical Infectious Diseases. 2003 Jun 1;36(11):e134–9.
62 Atzmony L, Halutz O, Avidor B, Finn T, Zimmerman O, Steinvil A, et al. Incidence of Cytomegalovirus-associated thrombosis and its risk factors: A case-control study. Thrombosis Research. 2010 Dec;126(6):e439–43.
63 Atzmony L, Grosfeld A, Saar N, Justo D. Inherited and acquired predispositions for thrombosis in immunocompetent patients with cytomegalovirus-associated thrombosis. European Journal of Internal Medicine. 2010 Feb;21(1):2–5.
64 Gueddi S, Righini M, Mezger N, Morard I, Kaiser L, Giostra E, et al. Portal vein thrombosis following a primary cytomegalovirus infection in an immunocompetent adult. Thromb Haemost. 2006;95(01):199–201.
65 Spahr L, Cerny A, Morard I, Rubbia-Brandt L, Schrenzel J. Acute partial Budd-Chiari syndrome and portal vein thrombosis in cytomegalovirus primary infection: a case report. BMC Gastroenterol. 2006 Mar 10;6:10.
66 Jeanneret C, Reutlinger C, Hirsch HH, Cathomas G, Rothen M, Martius F, et al. Multifocal thrombophlebitis and deep vein thrombosis in primary cytomegalovirus infection – A histopathological analysis. Thromb Haemost. 2008;99(02):445–7.
67 Kelkar AH, Jacob KS, Yousif EB, Farrell JJ. Venous thromboembolism related to cytomegalovirus infection. Medicine (Baltimore). 2017 Dec 22;96(51).
68 Squizzato A, Ageno W, Cattaneo A, Brumana N. A Case Report and Literature Review of Portal Vein Thrombosis Associated with Cytomegalovirus Infection in Immunocompetent Patients. Clinical Infectious Diseases. 2007 Jan 15;44(2):e13–6.
69 Mashav N, Saar N, Chundadze T, Steinvil A, Justo D. Epstein-Barr virus-associated venous thromboembolism: A case report and review of the literature. Thrombosis Research. 2008 Jan;122(4):570–1.
70 Galli L, Gerdes VEA, Guasti L, Squizzato A. Thrombosis Associated with Viral Hepatitis. J Clin Transl Hepatol. 2014 Dec;2(4):234–9.
71 Harms PW, Schmidt LA, Smith LB, Newton DW, Pletneva MA, Walters LL, et al. Autopsy Findings in Eight Patients With Fatal H1N1 Influenza. Am J Clin Pathol. 2010 Jul;134(1):27–35.
72 Mauad T, Hajjar LA, Callegari GD, da Silva LFF, Schout D, Galas FRBG, et al. Lung Pathology in Fatal Novel Human Influenza A (H1N1) Infection. Am J Respir Crit Care Med. 2010 Jan;181(1):72–9.
73 Avnon LS, Munteanu D, Smoliakov A, Jotkowitz A, Barski L. Thromboembolic events in patients with severe pandemic influenza A/H1N1. European Journal of Internal Medicine. 2015 Oct;26(8):596–8.
74 Zhu T, Carcaillon L, Martinez I, Cambou J-P, Kyndt X, Guillot K, et al. Association of influenza vaccination with reduced risk of venous thromboembolism. Thromb Haemost. 2009;102(12):1259–64.
75 Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Hepatitis C Virus Infection and Risk of Venous Thromboembolism: A Systematic Review and Meta-Analysis. Ann Hepatol. 2017 Aug;16(4):514–20.
76 Oliveira GN, Basso S, Sevivas T, Neves N. Varicella complicated by cellulitis and deep vein thrombosis. BMJ Case Rep. 2017 Sep 7;2017.
77 Rabah F, El-Banna N, Abdel-Baki M, Beshlavi I, Macaraig D, Bhuyan D, et al. Postvaricella Thrombosis—Report of Two Cases and Literature Review: The Pediatric Infectious Disease Journal. 2012 Sep;31(9):985–7.
78 Gogos CA, Apostolidou E, Bassaris HP, Vagenakis AG. Three cases of varicella thrombophlebitis as a complication of varicella zoster virus infection. Eur J Clin Microbiol Infect Dis. 1993 Jan;12(1):43–5.
79 Ahonkhai AA, Gebo KA, Streiff MB, Moore RD, Segal JB. Venous Thromboembolism in Patients With HIV/AIDS A Case-Control Study. J Acquir Immune Defic Syndr. 2008 Jul 1;48(3):310–4.
80 Rasmussen LD, Dybdal M, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, et al. HIV and risk of venous thromboembolism: a Danish nationwide population-based cohort study. HIV Medicine. 2011;12(4):202–10.
81 Alvaro-Meca A, Ryan P, Martínez-Larrull E, Micheloud D, Berenguer J, Resino S. Epidemiological trends of deep venous thrombosis in HIV-infected subjects (1997–2013): A nationwide population-based study in Spain. European Journal of Internal Medicine. 2018 Feb 1;48:69–74.
82 Dentali F, Nicolini E, Ageno W. Venous and Arterial Thrombosis Associated with HIV Infection. Semin Thromb Hemost. 2012 Jul;38(05):524–9.
83 Klein SK, Slim EJ, de Kruif MD, Keller TT, Brandjes DPM. Is chronic HIV infection associated with venous thrombotic disease? A systematic review. 2005;63(4):8.
84 Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Apr;18(4):844–7.
85 Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Apr 10;
86 Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 Apr 9;
87 Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020 23;382(17):e38.
88 Wilson Dib R, Chaftari A-M, Hachem RY, Yuan Y, Dandachi D, Raad II. Catheter-Related Staphylococcus aureus Bacteremia and Septic Thrombosis: The Role of Anticoagulation Therapy and Duration of Intravenous Antibiotic Therapy. Open Forum Infectious Diseases. 2018 Oct 1;5(10).
89 Cahill TJ, Prendergast BD. Infective endocarditis. The Lancet. 2016 Feb 27;387(10021):882–93.
90 Andes DR, Urban AW, Acher CW, Maki DG. Septic thrombosis of the basilic, axillary, and subclavian veins caused by a peripherally inserted central venous catheter. The American Journal of Medicine. 1998 Nov 1;105(5):446–50.
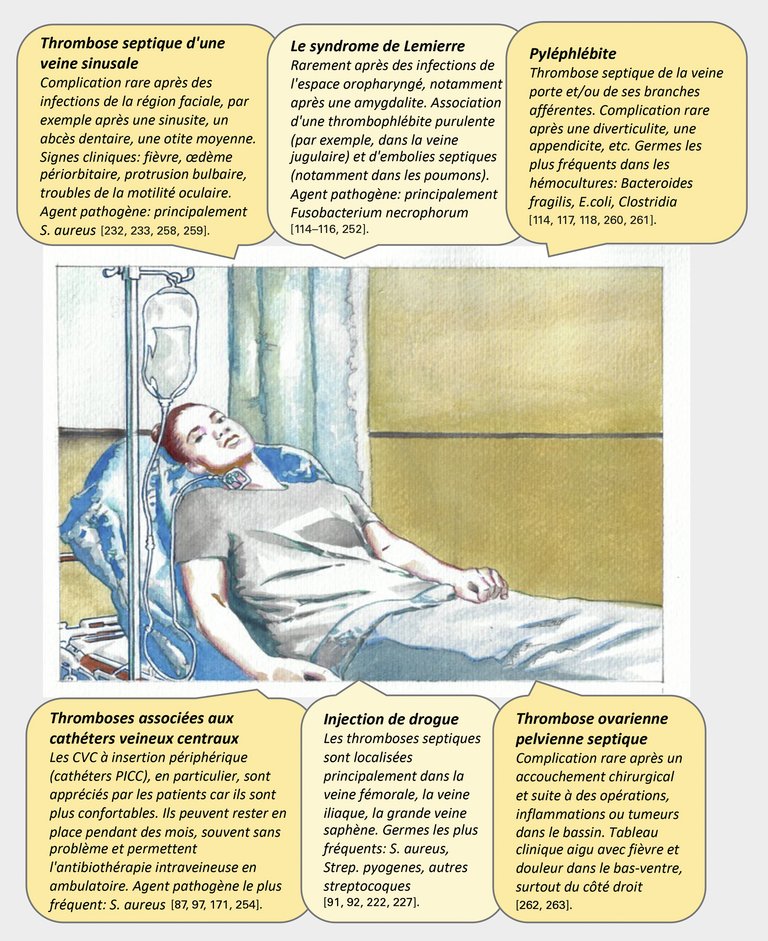
91 Dalager-Pedersen M, Søgaard M, Schønheyder HC, Thomsen RW, Baron JA, Nielsen H. Venous Thromboembolism after Community-Acquired Bacteraemia: A 20-year Danish Cohort Study. Salluh JIF, editor. PLoS ONE. 2014 Jan 23;9(1):e86094.
92 Fäh F, Zimmerli W, Jordi M, Schoenenberger RA. Septic deep venous thrombosis in intravenous drug users. SWISS MED WKLY. :8.
93 Mertz D, Khanlari B, Viktorin N, Battega M, Fluckiger U. Less than 28 Days of Intravenous Antibiotic Treatment Is Sufficient for Suppurative Thrombophlebitis in Injection Drug Users. Clinical Infectious Diseases. 2008 Mar 1;46(5):741–4.
94 Liesenborghs L, Verhamme P, Vanassche T. Staphylococcus aureus, master manipulator of the human hemostatic system. Journal of Thrombosis and Haemostasis. 2018;16(3):441–54.
95 Martin E, Cevik C, Nugent K. The role of hypervirulent Staphylococcus aureus infections in the development of deep vein thrombosis. Thrombosis Research. 2012 Sep;130(3):302–8.
96 Peetermans M, Verhamme P, Vanassche T. Coagulase Activity by Staphylococcus aureus: A Potential Target for Therapy? Semin Thromb Hemost. 2015 May 14;41(04):433–44.
97 Keene AB. Thrombosis in central catheter–associated Staphylococcus aureus bacteremia: Always scan the site?*: Critical Care Medicine. 2008 Feb;36(2):618–9.
98 Crowley AL, Peterson GE, Benjamin DK, Rimmer SH, Todd C, Cabell CH, et al. Venous thrombosis in patients with short- and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med. 2008 Feb;36(2):385–90.
99 Snyder GM, Pothuru S. Cavernous Sinus Thrombosis Associated With MRSA Bacteremia. The American Journal of the Medical Sciences. 2008 Oct;336(4):353.
100 Gosbell IB. Diagnosis and management of catheter-related bloodstream infections due to Staphylococcus aureus. Intern Med J. 2005 Dec;35(s2):S45–62.
101 Beckert L, Rahman A. Pneumonia and venous thromboembolism: Is the evidence catching up with the guidelines? Respirology. 2015;20(5):695–6.
102 Geelen S, Bhattacharyya C, Tuomanen E. Induction of procoagulant activity on human endothelial cells by Streptococcus pneumoniae. Infection and Immunity. 1992 Oct;60(10):4179.
103 Schut ES, Brouwer MC, de Gans J, Florquin S, Troost D, van de Beek D. Delayed cerebral thrombosis after initial good recovery from pneumococcal meningitis. Neurology. 2009 Dec 8;73(23):1988–95.
104 Odeh M, Pick N, Oliven A, Odeh M. Deep Venous Thrombosis Associated with Acute Brucellosis: A Case Report. Angiology. 2000 Mar;51(3):253–6.
105 Sanchez-Gonzalez J, Garcia-Delange T, Martos F, Colmenero JD. Thrombosis of the abdominal aorta secondary toBrucella spondylitis. Infection. 1996 May;24(3):261–2.
106 Koubaa M, Frigui M, Cherif Y, Jallouli M, Kaddour N, Ben Jemaa M, et al. Deep vein thrombosis associated with acute brucellosis: a case report and review of the literature. Korean J Intern Med. 2013 Sep;28(5):628–30.
107 Tolaj I, Mehmeti M, Ramadani H, Tolaj J, Dedushi K, Fejza H. Brucellosis Associated with Deep Vein Thrombosis. Infect Dis Rep. 2014 Nov 19;6(4).
108 Memish ZA, Bannatyne RM, Alshaalan M. Endophlebitis of the Leg Caused by Brucella Infection. Journal of Infection. 2001 Jul;42(4):281–3.
109 Davoudi AR, Tayebi A, Najafi N, Kasiri E. Deep vein thrombosis as a rare complication of brucellosis. Caspian J Intern Med. 2014;5(2):127–9.
110 Kechaou I, Cherif E, Ben Hassine L, Khalfallah N. Deep vein thrombosis and tuberculosis: a causative link? BMJ Case Rep. 2014 May 23;2014.
111 Robson SC, White NW, Aronson I, Woollgar R, Goodman H, Jacobs P. Acute-phase response and the hypercoagulable state in pulmonary tuberculosis. British Journal of Haematology. 1996 Jun;93(4):943–9.
112 Ambrosetti M, Ferrarese M, Codecasa LR, Besozzi G, Sarassi A, Viggiani P, et al. Incidence of Venous Thromboembolism in Tuberculosis Patients. Respiration. 2006;73(3):396–396.
113 Koster T, Rosendaal FR, Lieuw-A-Len DD, Kroes ACM, Emmerich JD, Dissel J van. Chlamydia pneumoniae IgG seropositivity and risk of deep-vein thrombosis. The Lancet. 2000 May 13;355(9216):1694–5.
114 Lozinguez O, Arnaud E, Belec L, Nicaud V, Alhenc-Gelas M, Fiessinger J-N, et al. Demonstration of an Association between Chlamydia pneumoniae Infection and Venous Thromboembolic Disease. Thromb Haemost. 2000;83(06):887–91.
115 Chirinos JA, Garcia J, Alcaide ML, Toledo G, Baracco GJ, Lichtstein DM. Septic Thrombophlebitis. Am J Cardiovasc Drugs. 2006 Jan 1;6(1):9–14.
116 Walkty A, Embil J. Lemierre’s Syndrome. N Engl J Med. 2019 Mar 21;380(12):e16.
117 Kuppalli K, Livorsi D, Talati NJ, Osborn M. Lemierre’s syndrome due to Fusobacterium necrophorum. Lancet Infect Dis. 2012 Oct;12(10):808–15.
118 Falkowski AL, Cathomas G, Zerz A, Rasch H, Tarr PE. Pylephlebitis of a variant mesenteric vein complicating sigmoid diverticulitis. Journal of Radiology Case Reports. 2014 Feb 27;8(2).
119 Plemmons RM, Dooley DP, Longfield RN. Septic Thrombophlebitis of the Portal Vein (Pylephlebitis): Diagnosis and Management in the Modern Era. Clinical Infectious Diseases. 1995 Nov 1;21(5):1114–20.
120 Levi M. New insights into pathways that determine the link between infection and thrombosis. 2012;70(3):7.
121 Levi M, van der Poll T. Inflammation and coagulation. Critical Care Medicine. 2010 Feb;38:S26.
122 van der Poll T, Boer JD de, Levi M. The effect of inflammation on coagulation and vice versa: Current Opinion in Infectious Diseases. 2011 Jun;24(3):273–8.
123 Dickson BC. Venous Thrombosis: On the History of Virchow’s Triad. University of Toronto Medical Journal. 2004;81(3):6.
124 Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). The American Journal of Medicine. 2002 Dec 1;113(8):636–42.
125 Jezovnik MK, Fareed J, Poredos P. Patients With a History of Idiopathic Deep Venous Thrombosis Have Long-Term Increased Levels of Inflammatory Markers and Markers of Endothelial Damage. Clinical and Applied Thrombosis/Hemostasis. 2017 Mar;23(2):124–31.
126 Abdel-Wahab N, Talathi S, Lopez-Olivo MA, Suarez-Almazor ME. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018 Apr;27(4):572–83.
127 Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almazor ME. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. 2016 Dec;25(14):1520–31.
128 Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. New England Journal of Medicine. 2020 Jul 16;383(3):288–90.
129 García-Carrasco M, Galarza-Maldonado C, Mendoza-Pinto C, Escarcega RO, Cervera R. Infections and the Antiphospholipid Syndrome. Clinical Reviews in Allergy & Immunology. 2009 Jun;36(2–3):104–8.
130 Tam K, Torres VJ. Staphylococcus aureus Secreted Toxins & Extracellular Enzymes. Microbiol Spectr. 2019 Mar;7(2).
131 Grundmeier M, Tuchscherr L, Brück M, Viemann D, Roth J, Willscher E, et al. Staphylococcal Strains Vary Greatly in Their Ability to Induce an Inflammatory Response in Endothelial Cells. The Journal of Infectious Diseases. 2010 Mar 15;201(6):871–80.
132 Sinha B, Herrmann M. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb Haemost. 2005 Jul 8;
133 Cervantes J, Rojas G. Virchow’s Legacy: Deep Vein Thrombosis and Pulmonary Embolism. World J Surg. 2005 Jun 1;29(1):S30–4.
134 Safavi-Abbasi S, Reis C, Talley MC, Theodore N, Nakaji P, Spetzler RF, et al. Rudolf Ludwig Karl Virchow: pathologist, physician, anthropologist, and politician: Implications of his work for the understanding of cerebrovascular pathology and stroke. Neurosurgical Focus. 2006 Jun 1;20(6):1–6.
135 Larena-Avellaneda A. Wirklich Virchows Trias? Gefässchirurgie. 2018 Mar;23(2):64–5.
136 Stanifer JW. Virchow’s triad: Kussmaul, Quincke and von Recklinghausen. J Med Biogr. 2016 Feb;24(1):89–100.
137 Dang TT, Majumdar SR, Marrie TJ, Eurich DT. Recurrent pneumonia: a review with focus on clinical epidemiology and modifiable risk factors in elderly patients. Drugs Aging. 2015 Jan;32(1):13–9.
138 Olson NC, Cushman M, Lutsey PL, McClure LA, Judd S, Tracy RP, et al. Inflammation markers and incident venous thromboembolism: the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. J Thromb Haemost. 2014 Dec;12(12):1993–2001.
139 Kunutsor SK, Seidu S, Blom AW, Khunti K, Laukkanen JA. Serum C-reactive protein increases the risk of venous thromboembolism: a prospective study and meta-analysis of published prospective evidence. Eur J Epidemiol. 2017;32(8):657–67.
140 Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis. n/a(n/a).
141 Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13;
142 Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 28;395(10229):1054–62.
143 Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. 2020 Apr 21;
144 Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 Apr 19;
145 Grandmaison G, Andrey A, Périard D, Engelberger RP, Carrel G, Doll S, et al. Systematic Screening for Venous Thromboembolic Events in COVID-19 Pneumonia. TH Open. 2020 Jun 8;4(2):e113–5.
146 Marik PE, Andrews L, Maini B. The incidence of deep venous thrombosis in ICU patients. Chest. 1997 Mar;111(3):661–4.
147 PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Cook D, Meade M, et al. Dalteparin versus unfractionated heparin in critically ill patients. The New England Journal of Medicine. 2011 Apr;364(14):1305-1314. DOI: 10.1056/nejmoa1014475. N Engl J Med. 2011 Apr 7;364(14):1305–14.
148 Patel R, Cook DJ, Meade MO, Griffith LE, Mehta G, Rocker GM, et al. Burden of Illness in venous ThromboEmbolism in Critical care: a multicenter observational study. Journal of Critical Care. 2005 Dec;20(4):341–7.
149 Lim W, Meade M, Lauzier F, Zarychanski R, Mehta S, Lamontagne F, Dodek P, McIntyre L, Hall R, Heels-Ansdell D, Fowler R, Pai M, Guyatt G, Crowther MA, Warkentin TE, Devereaux PJ, Walter SD, Muscedere J, Herridge M, Turgeon AF, Geerts W, Finfer S, Jacka M, Berwanger O, Ostermann M, Qushmaq I, Friedrich JO, Cook DJ; PROphylaxis for ThromboEmbolism in Critical Care Trial Investigators. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients*. Crit Care Med. 2015 Feb;43(2):401-10.
150 Hanify JM, Dupree LH, Johnson DW, Ferreira JA. Failure of chemical thromboprophylaxis in critically ill medical and surgical patients with sepsis. Journal of Critical Care. 2017 Feb;37:206–10.
151 Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2020 Jun 16;75(23):2950–73.
152 Casini A, Alberio L, Angelillo-Scherrer A, Fontana P, Gerber B, Graf L, et al. Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19 - a Swiss consensus statement by the Working Party Hemostasis. Swiss Med Wkly. 2020 06;150:w20247.
153 Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. Journal of Thrombosis and Thrombolysis. 2020 Jul;50(1):72–81.
154 Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020 Apr 30;
155 Condliffe R, Bunclark K, Hurdman J, Kiely D, MacLean R, Price L, Valerio C, Wort J,. British Thoracic Society. British Thoracic Society Guidance on Venous Thromboembolic Disease in patients with COVID-19. 2020;
156 Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020 Apr 28;1–3.
157 Busch MH, Timmermans SAMEG, Nagy M, Visser M, Huckriede J, Aendekerk JP, et al. Neutrophils and Contact Activation of Coagulation as Potential Drivers of COVID-19. Circulation. 2020 Nov 3;142(18):1787–90.
158 Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020 Sep 22;142(12):1176–89.
159 Middleton EA, He X-Y, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020 Sep 3;136(10):1169–79.
160 Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. Journal of Experimental Medicine. 2020 Jun 1;217(6):e20200652.
161 Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. Journal of Experimental Medicine. 2020 Dec 7;217(12):e20201129.
162 Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18(4):786–7.
163 Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 May 4;1–10.
164 Devreese KMJ, Linskens EA, Benoit D, Peperstraete H. Antiphospholipid antibodies in patients with COVID‐19: A relevant observation? Journal of Thrombosis and Haemostasis. 2020 Sep;18(9):2191–201.
165 Borghi MO, Beltagy A, Garrafa E, Curreli D, Cecchini G, Bodio C, et al. Anti-Phospholipid Antibodies in COVID-19 Are Different From Those Detectable in the Anti-Phospholipid Syndrome. Frontiers in Immunology. 2020 Oct 15;11.
166 Pineton de Chambrun M, Frere C, Miyara M, Amoura Z, Martin-Toutain I, Mathian A, et al. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? Journal of Internal Medicine. 2020 Jul 13;
167 Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. Journal of Thrombosis and Haemostasis. 2020 Aug;18(8):2064–5.
168 Siguret V, Voicu S, Neuwirth M, Delrue M, Gayat E, Stépanian A, et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thrombosis Research. 2020 Nov;195:74–6.
169 Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Science Translational Medicine. 2020 Nov 18;12(570):eabd3876.
170 Cohen H, Efthymiou M, Isenberg DA. Use of direct oral anticoagulants in antiphospholipid syndrome. J Thromb Haemost. 2018 Jun;16(6):1028–39.
171 Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. n/a(n/a).
172 Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. 2020 May 6;
173 Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 Apr 20;
174 Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020 Apr 20;
175 Breakey N, Escher R. D-dimer and mortality in COVID-19: a self-fulfilling prophecy or a pathophysiological clue? Swiss Medical Weekly. 2020 May 26;150(2122).
176 Beeching N, Fletcher T, Fowler R et al. Coronavirus disease 2019 (COVID-19) Straight to the point of care. BMJ Best Practice. 2020 Dec.
177 Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With COVID-19. Chest. 2020 Jun;
178 Bikdeli B, Talasaz AH, Rashidi F, Sharif-Kashani B, Farrokhpour M, Bakhshandeh H, et al. Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: Rationale and design of the INSPIRATION/INSPIRATION-S studies. Thrombosis Research. 2020 Dec;196:382–94.
179 Tritschler T, Mathieu M, Skeith L, Rodger M, Middeldorp S, Brighton T, et al. Anticoagulant interventions in hospitalized patients with COVID-19: A scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020 Nov;18(11):2958–67.
180 Fontana P, Casini A, Robert-Ebadi H, Glauser F, Righini M, Blondon M. Venous thromboembolism in COVID-19: systematic review of reported risks and current guidelines. Swiss Medical Weekly. 2020 Jun 22;150(2526).
181 Oldenburg J ,Klamroth R, Langer F, von Auer C, Albisetti M, Ay C, Korte W, Aktualisierte Empfehlungen zur Thromboseprophylaxe bei SARS-CoV-2 (COVID-19) der deutschen Gesellschaft für Thrombose und Hämostaseforschung [Internet].
182 Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With COVID-19. Chest [Internet]. 2020 Jun
183 Mendoza E. COVID-19 Early Stage Disease Progression and Anticoagulants: Investigation Rationale, Challenges and Difficulties. Phlebologie. 2020 Aug;49(4):199–203.
184 Kucher N,. Studie zur Verhinderung von Blutgerinnseln mit Clexane® bei ambulanten Patienten mit Coronavirus-Infektion [Internet]. Kofam | Studienregister SNCTP. [cited 2020 Sep 19]. Available from: https://www.kofam.ch/de/studienportal/suche/94819/studie/52205
185 Patell R, Bogue T, Koshy A, Bindal P, Merrill M, Aird WC, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020 Sep 10;136(11):1342–6.
186 Roberts LN, Whyte MB, Georgiou L, Giron G, Czuprynska J, Rea C, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020 Sep 10;136(11):1347–50.
187 Folsom AR, Lutsey PL, Astor BC, Cushman M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost. 2009 Oct;102(4):615–9.
188 Zacho J, Tybjærg-Hansen A, Nordestgaard BG. C-Reactive Protein and Risk of Venous Thromboembolism in the General Population. ATVB. 2010 Aug;30(8):1672–8.
189 Poredos P, Jezovnik MK. The role of inflammation in venous thromboembolism and the link between arterial and venous thrombosis. Int Angiol. 2007 Dec;26(4):306–11.
190 Musher DM, Abers MS, Corrales-Medina VF. Acute Infection and Myocardial Infarction. Longo DL, editor. New England Journal of Medicine. 2019 Jan 10;380(2):171–6.
191 Horvei LD, Grimnes G, Hindberg K, Mathiesen EB, Njølstad I, Wilsgaard T, et al. C-reactive protein, obesity, and the risk of arterial and venous thrombosis. J Thromb Haemost. 2016;14(8):1561–71.
192 Hald EM, Brækkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Brox J, et al. High-sensitivity C-reactive protein is not a risk factor for venous thromboembolism: the Tromsø study. Haematologica. 2011 Aug 1;96(8):1189–94.
193 Lippi G, Favaloro EJ, Montagnana M, Franchini M. C-reactive protein and venous thromboembolism: causal or casual association? Clinical Chemistry and Laboratory Medicine. 2010;48(12):1693–701.
194 Quist-Paulsen P, Naess IA, Cannegieter SC, Romundstad PR, Christiansen SC, Rosendaal FR, et al. Arterial cardiovascular risk factors and venous thrombosis: results from a population-based, prospective study (the HUNT 2). Haematologica. 2010 Jan 1;95(1):119–25.
195 Grimnes G, Isaksen T, Tichelaar YIGV, Brox J, Brækkan SK, Hansen J-B. C-reactive protein and risk of venous thromboembolism: results from a population-based case-crossover study. Haematologica. 2018 Jul;103(7):1245–50.
196 Opal SM, Cohen J. Clinical Gram-positive sepsis. Critical Care Medicine. 1999 Aug 1;27(8):1608–16.
197 Rivera J, Vannakambadi G, Höök M, Speziale P. Fibrinogen-binding proteins of Gram-positive bacteria. Thromb Haemost. 2007;98(09):503–11.
198 Yeaman MR, Bayer AS. Staphylococcus aureus, platelets, and the heart. Curr Infect Dis Rep. 2000 Jul;2(4):281–98.
199 Nendaz M, Spirk D, Kucher N, Aujesky D, Hayoz D, Beer JH, et al. Multicentre validation of the Geneva Risk Score for hospitalised medical patients at risk of venous thromboembolism. Explicit ASsessment of Thromboembolic RIsk and Prophylaxis for Medical PATients in SwitzErland (ESTIMATE). Thromb Haemost. 2014 Mar 3;111(3):531–8.
200 Blondon M, Spirk D, Kucher N, Aujesky D, Hayoz D, Beer JH, et al. Comparative Performance of Clinical Risk Assessment Models for Hospital-Acquired Venous Thromboembolism in Medical Patients. Thromb Haemost. 2018;118(1):82–9.
201 Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):338S-400S.
202 Cohen AT, Alikhan R, Arcelus JI, Bergmann J-F, Haas S, Merli GJ, et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost [Internet]. 2005 Sep 9
203 Dessole S, Capobianco G, Arru A, Demurtas P, Ambrosini G. Postpartum ovarian vein thrombosis: an unpredictable event: two case reports and review of the literature. Arch Gynecol Obstet. 2003 Feb;267(4):242–6.
204 Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009 Jul;49(1):1–45.
205 Musher DM, Rueda AM, Kaka AS, Mapara SM. The Association between Pneumococcal Pneumonia and Acute Cardiac Events. Clin Infect Dis. 2007 Jul 15;45(2):158–65.
206 Stein PD, Afzal A, Henry JW, Villareal CG. Fever in Acute Pulmonary Embolism. CHEST. 2000 Jan 1;117(1):39–42.
207 Stein PD, Terrin ML, Hales CA, Palevsky HI, Saltzman HA, Thompson BT, et al. Clinical, Laboratory, Roentgenographic, and Electrocardiographic Findings in Patients with Acute Pulmonary Embolism and No Pre-Existing Cardiac or Pulmonary Disease. CHEST. 1991 Sep 1;100(3):598–603.
208 Saad M, Shaikh DH, Mantri N, Alemam A, Zhang A, Adrish M. Fever is associated with higher morbidity and clot burden in patients with acute pulmonary embolism. BMJ Open Respir Res. 2018;5(1):e000327.
209 Calvo-Romero JM, Lima-Rodríguez EM, Pérez-Miranda M, Bureo-Dacal P. Low-grade and high-grade fever at presentation of acute pulmonary embolism. Blood Coagul Fibrinolysis. 2004 Jun;15(4):331–3.
210 Watanakunakorn C, Hayek F. High fever (greater than 39 degrees C) as a clinical manifestation of pulmonary embolism. Postgrad Med J. 1987 Nov;63(745):951–3.
211 Saad M, Shaikh DH, Adrish M. A rare case report of a saddle pulmonary embolism presenting with high grade fevers, responsive to anticoagulation. Medicine (Baltimore). 2018 Mar;97(9):e0002.
212 Murray HW, Ellis GC, Blumenthal DS, Sos TA. Fever and pulmonary thromboembolism. Am J Med. 1979 Aug;67(2):232–5.
213 Aburahma AF, Saiedy S. Deep vein thrombosis as probable cause of fever of unknown origin. W V Med J. 1997 Feb;93(1):368–70.
214 Mourad O, Palda V, Detsky AS. A Comprehensive Evidence-Based Approach to Fever of Unknown Origin. Arch Intern Med. 2003 Mar 10;163(5):545–51.
215 AbuRahma AF, Saiedy S, Robinson PA, Boland JP, Cottrell DJ, Stuart C. Role of venous duplex imaging of the lower extremities in patients with fever of unknown origin. Surgery. 1997 Apr;121(4):366–71.
216 Barba R, Di Micco P, Blanco-Molina Á, Delgado C, Cisneros E, Villalta J, et al. Fever and deep venous thrombosis. Findings from the RIETE registry. J Thromb Thrombolysis. 2011 Oct 1;32(3):288–92.
217 Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet. 1998 May 16;351(9114):1467–71.
218 Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011 Jun;8(6):e1001048.
219 Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang C-CH, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015 Jan 20;313(3):264–74.
220 Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012 Feb 14;125(6):773–81.
221 Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias LA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000 Jul;79(4):210–21.
222 Smeeth L, Hubbard R. Risk of Myocardial Infarction and Stroke after Acute Infection or Vaccination. The New England Journal of Medicine. 2004;8.
223 Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003 Apr 3;348(14):1322–32.
224 Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation. 2002 May 7;105(18):2143–7.
225 Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar Adeeba, Naguib S, et al. Influenza Infection Exerts Prominent Inflammatory and Thrombotic Effects on the Atherosclerotic Plaques of Apolipoprotein E–Deficient Mice. Circulation. 2003 Feb 11;107(5):762–8.
226 Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. New England Journal of Medicine. 2018 Jan 25;378(4):345–53.
227 Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. The Lancet Infectious Diseases. 2010 Feb;10(2):83–92.
228 Dalager-Pedersen M, Søgaard M, Schønheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014 Apr 1;129(13):1387–96.
229 Santos-Gallego CG, Badimon JJ. The sum of two evils: pneumonia and myocardial infarction: is platelet activation the missing link? J Am Coll Cardiol. 2014 Nov 4;64(18):1926–8.
230 Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al. Atrial Fibrillation and the Risk of Myocardial Infarction. JAMA Intern Med. 2014 Jan;174(1):107–14.
231 Klein Klouwenberg PMC, Frencken JF, Kuipers S, Ong DSY, Peelen LM, van Vught LA, et al. Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am J Respir Crit Care Med. 2016 Jul 28;195(2):205–11.
232 Bosch NA, Cimini J, Walkey AJ. Atrial Fibrillation in the ICU. Chest. 2018 Dec;154(6):1424–34.
233 Anné W, Willems R, Roskams T, Sergeant P, Herijgers P, Holemans P, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. 2005 Sep 1;67(4):655–66.
234 Chimenti C, Russo MA, Carpi A, Frustaci A. Histological substrate of human atrial fibrillation. Biomedicine & Pharmacotherapy. 2010 Mar;64(3):177–83.
235 Nguyen BL, Fishbein MC, Chen LS, Chen P-S, Masroor S. Histopathological Substrate For Chronic Atrial Fibrillation in Humans. Heart Rhythm. 2009 Apr;6(4):454–60.
236 Gandhi S, Litt D, Narula N. New-onset atrial fibrillation in sepsis is associated with increased morbidity and mortality. Neth Heart J. 2015 Feb;23(2):82–8.
237 Keller M, Meierhenrich R. [New onset atrial fibrillation in patients with sepsis]. Anaesthesist. 2017 Oct;66(10):786–94.
238 Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, Voglic S, Bögelein D, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14(3):R108.
239 Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation. 2003 Dec 16;
240 Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001 Dec 11;104(24):2886–91.
241 Harada M, Van Wagoner DR, Nattel S. Role of Inflammation in Atrial Fibrillation Pathophysiology and Management. Circ J. 2015;79(3):495–502.
242 Issac TT, Dokainish H, Lakkis NM. Role of Inflammation in Initiation and Perpetuation of Atrial Fibrillation: A Systematic Review of the Published Data. Journal of the American College of Cardiology. 2007 Nov 20;50(21):2021–8.
243 Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident Stroke and Mortality Associated with New-onset Atrial Fibrillation in Patients Hospitalized with Severe Sepsis. JAMA. 2011 Nov 23;306(20):2248–54.
244 Watson T, Shantsila E, Lip GYH. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009 Jan 10;373(9658):155–66.
245 Khan AA, Lip GYH. The prothrombotic state in atrial fibrillation: pathophysiological and management implications. Cardiovascular Research. 2019 Jan 1;115(1):31–45.
246 Choudhury A, Lip GYH. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol Haemost Thromb. 2003 Dec;33(5–6):282–9.
247 Chean CS, McAuley D, Gordon A, Welters ID. Current practice in the management of new-onset atrial fibrillation in critically ill patients: a UK-wide survey. PeerJ. 2017 Sep 8;5.
248 Verma A. Does “Secondary” Atrial Fibrillation Really Exist? JACC: Clinical Electrophysiology. 2018 Mar;4(3):394–6.
249 Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal. 2016 Oct 7;37(38):2893–962.
250 January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019 Jul 9;140(2).
251 Lubitz S. A., Yin X., Rienstra M., Schnabel RB., Walkey Allan J., Magnani JW., et al. Long-Term Outcomes of Secondary Atrial Fibrillation in the Community. Circulation. 2015 May 12;131(19):1648–55.
252 Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994 Jan;89(1):224–7.
253 Falagas ME, Vardakas KZ, Athanasiou S. Intravenous heparin in combination with antibiotics for the treatment of deep vein septic thrombophlebitis: A systematic review. European Journal of Pharmacology. 2007 Feb;557(2–3):93–8.
254 Gall CP, Schiemann U, Schmidli J, Sollinger D, Widmer MK. Septische Cavathrombose. :2.
255 Kniemeyer H, Grabitz K, Buhl R, Wust H, Sandmann W. Surgical treatment of septic deep venous thrombosis. Surgery. 1995 Jul;118(1):49–53.
256 Tomford JW, Hershey CO, Mclaren CE, Porter DK, Cohen DI, Adams AP. Intravenous Therapy Team and Peripheral Venous Catheter-Associated Complications: A Prospective Controlled Study. Survey of Anesthesiology. 1985 Apr;29(2):122.
257 Tagalakis V, Kahn SR, Libman M, Blostein M. The epidemiology of peripheral vein infusion thrombophlebitis: a critical review. The American Journal of Medicine. 2002 Aug;113(2):146–51.
258 Scheidegger C, Zimmerli W. Infectious complications in drug addicts: seven-year review of 269 hospitalized narcotics abusers in Switzerland. Rev Infect Dis. 1989 Jun;11(3):486–93.
259 Mackenzie A, Laing R, Douglas J, Greaves M, Smith C. High prevalence of iliofemoral venous thrombosis with severe groin infection among injecting drug users in North East Scotland: successful use of low molecular weight heparin with antibiotics. Postgrad Med J. 2000 Sep;76(899):561–5.
260 McColl MD, Tait RC, Greer IA, Walker ID. Injecting drug use is a risk factor for deep vein thrombosis in women in Glasgow. British Journal of Haematology. 2001;112(3):641–3.
261 Kwiatkowska W, Knysz B, Gąsiorowski J, Witkiewicz W. Deep vein thrombosis of the lower limbs in intravenous drug users. Postepy Hig Med Dosw (Online). 2015 Apr 22;69:510–20.
262 Ghanem G, Boktour M, Warneke C, Pham-Williams T, Kassis C, Bahna P, et al. Catheter-Related Staphylococcus aureus Bacteremia in Cancer Patients: High Rate of Complications With Therapeutic Implications. Medicine. 2007 Jan 1;86(1):54–60.
263 Baker CC, Francisco S, Petersen SR, Francisco S, Sheldon F, Francisco S. Septic Phlebitis: A Neglected Disease. The American Journal of Surgery. :7.
264 Choudhry AJ, Baghdadi YMK, Amr MA, Alzghari MJ, Jenkins DH, Zielinski MD. Pylephlebitis: a Review of 95 Cases. J Gastrointest Surg. 2016 Mar;20(3):656–61.
265 van der Poel NA, Mourits MP, de Win MML, Coutinho JM, Dikkers FG. Prognosis of septic cavernous sinus thrombosis remarkably improved: a case series of 12 patients and literature review. Eur Arch Otorhinolaryngol. 2018;275(9):2387–95.
266 Khatri IA, Wasay M. Septic cerebral venous sinus thrombosis. J Neurol Sci. 2016 Mar 15;362:221–7.
267 Bhatia K, Jones NS. Septic cavernous sinus thrombosis secondary to sinusitis: are anticoagulants indicated? A review of the literature. J Laryngol Otol. 2002 Sep;116(9):667–76.
268 Cohen Y, Tit A, Brauner M, Fosse J-P, Hoang P. Contribution of computerized tomography to the diagnosis of catheter-induced septic central venous thrombosis. Intensive Care Med. 1996 Nov;22(11):1279–80.
269 Johannesen KM, Bodtger U. Lemierre’s syndrome: current perspectives on diagnosis and management. Infect Drug Resist. 2016 Sep 14;9:221–7.
270 Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med. 1995 Jun 12;155(11):1161–6.
271 Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015 Oct 13;132(15):1435–86.
272 Deprèle C, Berthelot Ph, Lemetayer F, Comtet C, Fresard A, Cazorla C, et al. Risk factors for systemic emboli in infective endocarditis. Clinical Microbiology and Infection. 2004 Jan;10(1):46–53.
273 Wang A, Gaca JG, Chu VH. Management Considerations in Infective Endocarditis: A Review. JAMA. 2018 Jul 3;320(1):72.
274 Habib G. Embolic Risk in Subacute Bacterial Endocarditis: Determinants and Role of Transesophageal Echocardiography. :9.
275 Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). European Heart Journal. 2015 Nov 21;36(44):3075–128.
276 García-Cabrera E, Fernández-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noureddine M, Plata A, et al. Neurological Complications of Infective Endocarditis. :13.
277 Hui FK, Bain M, Obuchowski NA, Gordon S, Spiotta AM, Moskowitz S, et al. Mycotic aneurysm detection rates with cerebral angiography in patients with infective endocarditis. J NeuroIntervent Surg. 2015 Jun;7(6):449–52.
278 Sandre RM, Shafran SD. Infective Endocarditis: Review of 135 Cases over 9 Years. Clinical Infectious Diseases. 1996 Feb 1;22(2):276–86.
279 Johnson JD, Raff MJ, Barnwell PA, Chun CH. Splenic abscess complicating infectious endocarditis. Arch Intern Med. 1983 May;143(5):906–12.
280 Rajasekhar A, Streiff MB. How I treat central venous access device–related upper extremity deep vein thrombosis. Blood. 2017 May 18;129(20):2727–36.
281 Goodacre S, Sutton AJ, Sampson FC. Meta-Analysis: The Value of Clinical Assessment in the Diagnosis of Deep Venous Thrombosis. Ann Intern Med. 2005 Jul 19;143(2):129.
282 Johanning JM, Franklin DP, Thomas DD, Elmore JR. D-dimer and calf circumference in the evaluation of outpatient deep venous thrombosis. Journal of Vascular Surgery. 2002 Nov;36(5):877–80.
283 Mertz et al. - 2008 - Less than 28 Days of Intravenous Antibiotic Treatm.pdf.
284 Snygg-Martin U, Gustafsson L, Rosengren L, Alsiö Å, Ackerholm P, Andersson R, et al. Cerebrovascular Complications in Patients with Left-Sided Infective Endocarditis Are Common: A Prospective Study Using Magnetic Resonance Imaging and Neurochemical Brain Damage Markers. Clinical Infectious Diseases. 2008 Jul;47(1):23–30.
285 Eilbert W, Singla N. Lemierre’s syndrome. Int J Emerg Med. 2013 Oct 23;6:40.
286 Wall C, Moore J, Thachil J. Catheter-related thrombosis: A practical approach. J Intensive Care Soc. 2016 May;17(2):160–7.
287 Sridhar DC, Abou-Ismail MY, Ahuja SP. Central venous catheter-related thrombosis in children and adults. Thrombosis Research. 2020 Mar 1;187:103–12.
288 Rasmussen RV, Snygg-Martin U, Olaison L, Buchholtz K, Larsen CT, Hassager C, et al. Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology. 2009;114(4):284–91.
289 Randhawa MS, Pile J, Gomes M. Can patients with infectious endocarditis be safely anticoagulated? Cleveland Clinic Journal of Medicine. 2016 Mar 1;83(3):169–71.
290 Kaufman J, Demas C, Stark K, Flancbaum L. Catheter-Related Septic Central Venous Thrombosis—Current Therapeutic Options. West J Med. 1986 Aug;145(2):200–3.
291 Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infectious Diseases. 2013 Dec;13(1).
292 Afzal MZ, Saleh MM, Razvi S, Hashmi H, Lampen R. Utility of Lower Extremity Doppler in Patients with Lower Extremity Cellulitis: A Need to Change the Practice? Southern Medical Journal. 2015;108(7):6.
293 Zaghdoudi I, Rezgui M, Zouaoui W, Marhbene T, Jendoubi A, El Fatimi R, et al. Incidence of Deep Venous Thrombosis in Patients with Erysipelas of the Leg: Prospective Study of 30 Cases in an Emergency Department. Pathophysiology of Haemostasis and Thrombosis. 2007;36(5):271–4.
294 Gunderson CG, Chang JJ. Risk of deep vein thrombosis in patients with cellulitis and erysipelas. Thrombosis Research. 2013 Sep;132(3):336–40.
295 Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014 Oct;134(4):846–50.
296 Mortazavi M, Samiee MM, Spencer FA. Incidence of deep vein thrombosis in erysipelas or cellulitis of the lower extremities: Incidence of DVT in lower leg erysipelas or cellulitis. International Journal of Dermatology. 2013 Mar;52(3):279–85.
297 Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14(4):210–4.
298 Bersier D, Bounameaux H. Cellulitis and deep vein thrombosis: a controversial association. Journal of Thrombosis and Haemostasis. 2003;1(4):867–8.
299 Söderberg M, Hedström U, Sjunnesson M, Lärfars G, Jorup-Rönström C. Initial symptoms in pulmonary embolism differ from those in pneumonia: a retrospective study during seven years. Eur J Emerg Med. 2006 Aug;13(4):225–9.
300 Cha S-I, Choi K-J, Shin K-M, Lim J-K, Yoo S-S, Lee J, et al. Clinical characteristics of pulmonary embolism with concomitant pneumonia. Blood Coagul Fibrinolysis. 2016 Apr;27(3):281–6.
301 Lucassen WAM, Kuijs-Augustijn M, Erkens PMG, Geersing G-J, Büller HR, van Weert HCPM. The additional value of the CRP test in patients in whom the primary care physician excluded pulmonary embolism. Eur J Gen Pract. 2013 Sep;19(3):143–9.
302 Tetsuhara K, Tsuji S, Uematsu S, Kamei K. Pulmonary Embolism Mimicking Infectious Pleuritis. Pediatr Emerg Care. 2018 Nov;34(11):e201–3.
303 Kelly J, Hunt BJ, Rudd A, Lewis RR. Pulmonary embolism and pneumonia may be confounded after acute stroke and may co-exist. Age Ageing. 2002 Jul;31(4):235–9.
304 Jacoby CG, Mindell HJ. Lobar Consolidation in Pulmonary Embolism. Radiology. 1976 Feb;118(2):287–90.
305 Elliott CG, Goldhaber SZ, Visani L, DeRosa M. Chest radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry. Chest. 2000 Jul;118(1):33–8.
306 Cranley JJ. The Diagnosis of Deep Venous Thrombosis: Fallibility of Clinical Symptoms and Signs. Arch Surg. 1976 Jan 1;111(1):34.
307 Anand SS, Wells PS, Hunt D, Brill-Edwards P, Cook D, Ginsberg JS. Does this patient have deep vein thrombosis? JAMA. 1998 Apr 8;279(14):1094–9.
308 Cho HJ, Dunn AS. The Value of Using Ultrasound to Rule Out Deep Vein Thrombosis in Cases of Cellulitis. Journal of Hospital Medicine. 2017 Apr 1;12(4):259–61.
309 Bannow BTS, Skeith L. Diagnosis and management of postpartum ovarian vein thrombosis. Hematology Am Soc Hematol Educ Program. 2017 08;2017(1):168–71.
310 Mazzolai L, Haesler E, Milesi I, Hayoz D. D-dimers testing is not recommended for the exclusion of deep vein thrombosis in outpatients with lower limb erysipelas. Thromb Haemost. 2002 Nov;88(5):880.
311 H’ng MWC, Loh SS, Earnest A, Wansaicheong GKL. Effectiveness of an algorithm in reducing the number of unnecessary ultrasound scans for deep vein thrombosis: an evaluation report. Singapore Med J 2012; 53(9):4.
Les quatre références les plus importantes
115 Chirinos JA, Garcia J, Alcaide ML, Toledo G, Baracco GJ, Lichtstein DM. Septic Thrombophlebitis. Am J Cardiovasc Drugs. 2006 Jan 1;6(1):9–14.
190 Musher DM, Abers MS, Corrales-Medina VF. Acute Infection and Myocardial Infarction. Longo DL, editor. New England Journal of Medicine. 2019 Jan 10;380(2):171–6.
204 Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009 Jul;49(1):1–45.
291 Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infectious Diseases. 2013 Dec;13(1).